ASOURCE®TIMES
政府が国策として医療DXを強力に推進する中、個々の医療機関にもオンライン資格確認等システムの導入を始め、次々と医療DXへの対応が求められています。2026年度には標準型電子カルテの本格運用が予定されており、医療機関には大きな戸惑いがみられます。そこで、標準型電子カルテの開発や普及の目的、それに伴う既存の電子カルテへの影響、今医療機関が対応すべきことなどを中心に解説します。

ご存知のように、国は医療DX(デジタルトランスフォーメーション)を推進しています。厚生労働省は「医療DX令和ビジョン2030厚生労働省推進チーム」を設置し、全国医療情報プラットフォームの創設、電子カルテ情報の標準化および標準型電子カルテの開発、診療報酬改定DXの3つの柱を中心に取組みを進めています。
最初に取り組まれた「オンライン資格確認等システム」の導入により、クラウドのデータベースと医療機関を紐づけることができました。次に「電子処方箋管理サービス」が導入され、情報の共有化が始まりました。そして現在、全国医療情報プラットフォームの基盤を構築し、「電子カルテ情報共有サービス」に乗り出そうとしています。
国が取組みを進める一方で、医療機関における電子カルテシステムの普及率は5割程度であり大病院での導入はほぼ完了しているものの、中小病院や診療所の2軒に1軒は未導入の状況です。国がめざす医療DXを実現するには標準化された電子カルテを100%普及することが大前提となるため、この状況を早急に解消する必要があります。そこで、取り組まれているのが標準型電子カルテの開発です。
標準型電子カルテの開発は、プロダクトオーナーであるデジタル庁から委託されている企業が複数の電子カルテメーカーの技術支援を受けながら進めています。スケジュールは2023年度に調査研究・仕様整理、2024年度にα版の調達・システム開発、2025年度にモデル事業(α版提供開始)を実施、2026年度に本格運用という計画になっています。
ただし、この開発によって既存の電子カルテシステムがまったく使用できなくなるわけではなく、現在開発中の標準型電子カルテのモデルが公開された後、各メーカーがその技術を流用し、既存の電子カルテシステムを改良した場合も国の基準を満たすものとして認められます〈図参照〉。
一方で、標準化されていない電子カルテは使えなくなる可能性があるため、すでに電子カルテを導入している医療機関も新しく買い替えなくてはなりません。その際には国が補助金を出すことが見込まれているものの、この原資は税金なので全額補助ではないようです。
そもそも標準型電子カルテを搭載した電子カルテシステムの改良あるいは開発には、莫大なコストがかかることが懸念されています。また、クラウド管理になるため、インターネットへの依存度が高くなり、サイバーテロ対策も一層厳重に行う必要があります。さらに国は3文書(健康診断結果報告書、診療情報提供書、退院時サマリー)6情報(傷病名、アレルギー、感染症、薬剤禁忌、検査、処方)が共有できる機能を搭載することを各メーカーに求めています。これらの不具合や要望に対処していくと、標準型電子カルテを搭載した電子カルテの改良費・開発費はおのずと高額になります。
このような状況を踏まえ、医療機関が今、対応すべきことは2つあります。まず電子カルテを導入していない医療機関は、標準型電子カルテの開発完了を待たずに、速やかに電子カルテに移行することが重要です。標準型電子カルテが義務化されると需要と供給のバランスが崩れ、電子カルテに移行したくてもメーカーの人手不足で対応してもらえなくなるおそれがあるからです。
一方、既に電子カルテを導入している医療機関は、メーカーと相談しながらオンライン資格確認等システムや電子処方箋管理サービスに順次対応していくことが必要です。
そして、上記のことをある程度整備したら、令和6年度診療報酬改定で新設された「医療DX推進体制整備加算(8点)」を算定することを推奨する専門家もいます。この加算では、電子カルテ情報共有サービスへの参加が施設基準の一つになっていますが、この電子カルテ情報共有サービスは近い将来、義務化される可能性が高いため、先取りしておくことが賢明だというのが算定することを推奨する大きな理由です。
医療DXを推進するうえで、全国津々浦々まで電子カルテを普及させることは国の重要なミッションです。個々の医療機関はその潮流から取り残されないよう、備えていくことが肝心です。
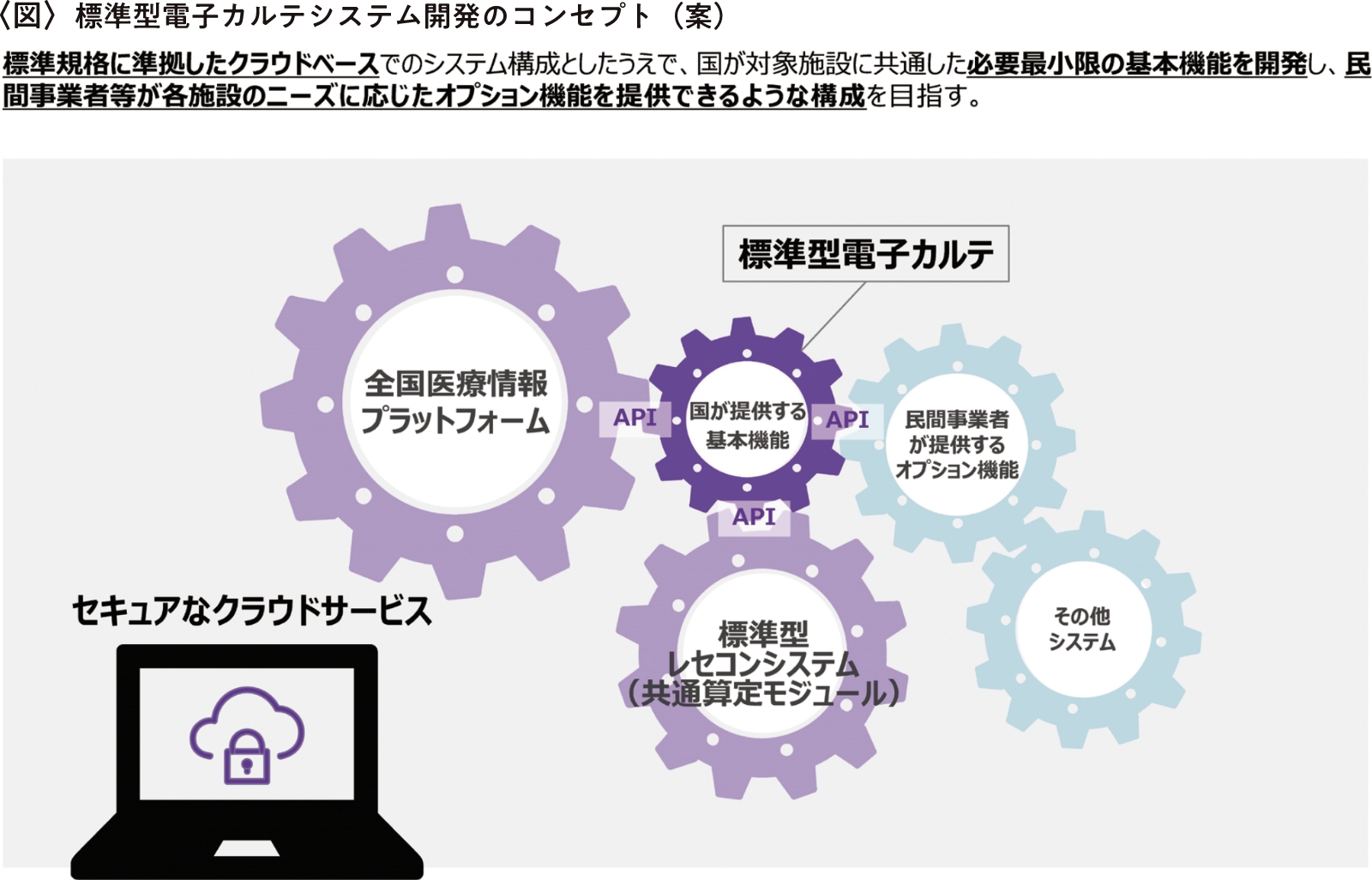
「第1回標準型電子カルテ検討ワーキンググループ資料」(厚生労働省)を加工して作成
(https://www.mhlw.go.jp/content/10808000/001178649.pdf)
取材先:大西大輔(MICTコンサルティング 代表取締役)