ASOURCE®TIMES

デジタルハリウッド大学大学院 特任教授
東京医科歯科大学 臨床教授
アイリス株式会社 共同創業者・取締役副社長CSO
加藤 浩晃
浜松医科大学医学部卒業後、眼科専門医として多くの手術を執刀、手術器具などの開発などを行う。2016年厚生労働省入省、臨床研究法制定やベンチャー支援政策を行う。退官後、オンライン診療や治療用アプリの事業開発に携わり、AI医療機器開発のアイリスを共同創業。『医療4.0』(日経BP社)など著書多数。一橋大学大学院 経営学修士(金融政策・経営財務MBA)。
医療のデジタル化の一環として注目される治療用アプリ。日本では現在3種が薬事承認されています。厚生労働省でベンチャー支援政策に携わり、自身も診断用や治療用のアプリの開発経験者で投資家でもある加藤浩晃先生に治療用アプリの特徴や未来について伺います。

医療分野で使用されるアプリは現在、さまざまなものが出ています。健康維持や病気の予防のために使う健康管理アプリと患者さん向けの疾患管理アプリがあり、疾患管理アプリには例えば血圧などを記録する症状記録アプリと、治療効果が証明されていて医療現場で処方される治療用アプリがあります(下表)。
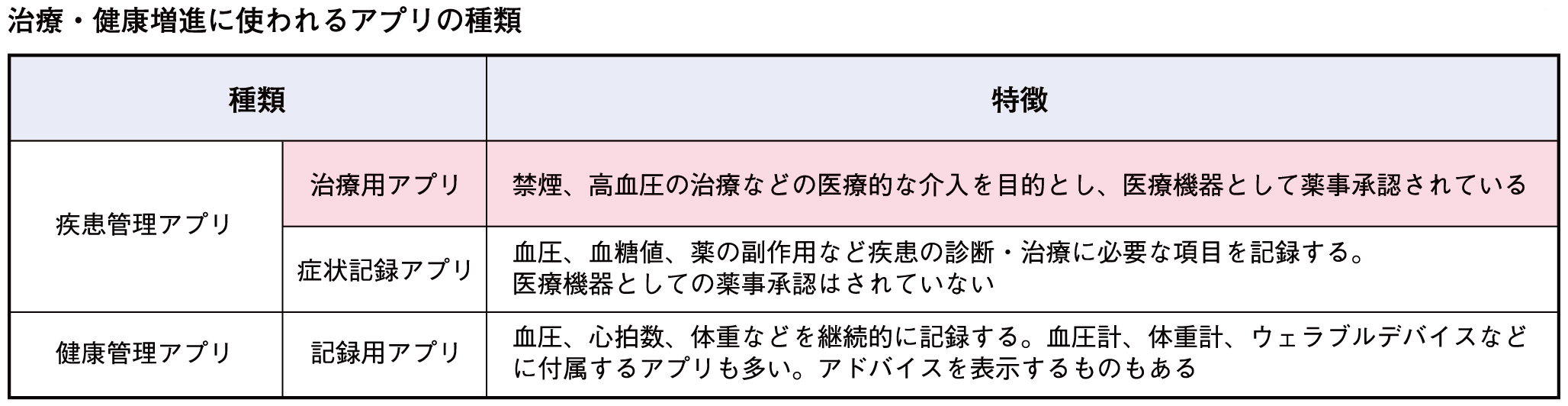
これらの区別は、アプリを出している企業の方針によります。治療用アプリは医薬品や医療機器と同様に薬事承認・保険適用というプロセスをたどって医師が処方します。一方、公的健康保険の適用や医師のアドバイスの有無にかかわらず、診療の支援ツールとして、患者さんが自由に使うのが症状記録アプリです。症状記録アプリには、がんの薬物療法の副作用を記録し、医療者とのコミュニケーションに使われるアプリなども含まれます。
現在、日本で薬事承認されている治療用アプリは「CureApp SC ニコチン依存症治療アプリ及びCOチェッカー」(2020年承認・保険適用)と高血圧治療補助アプリ「CureApp HT」(2022年承認・保険適用)、「サスメド Med CBT-i 不眠障害用アプリ」(2023年承認) の3つです。なお、「治療アプリⓇ」はCureApp社が商標登録しています。この記事内ではそれと区別して「治療用アプリ」の呼称でお話しします。
治療用アプリの良い点は、“診療”と“生活の場”をつなぐことです。医師は診療で処方した薬を患者さんがきちんと飲んでいるのかを正確に確認することはできませんし、患者さんも診療時間外は相談ができません。治療用アプリはその医療が及ばない、患者さんの普段の生活の場で力を発揮します。
例えば、「CureApp SC ニコチン依存症治療アプリ及びCOチェッカー」は禁煙補助薬バレニクリンとの併用で処方されるため、患者さんは必ず禁煙外来を受診します。そして、医師の前では禁煙する気持ちになるけれど、家で一人になるとタバコを吸いたくなる、そのときにアプリはチャットで支援してくれます。禁煙状況を入力した記録を見ながら、どんなときに喫煙したくなるのかを知ることもできます。医師も患者さんの記録を見て助言します。言わば、家にも医師や家庭教師がいるようなものです。
CureAppの治療用アプリの臨床試験は、治療の肝となる部分が入っていないコントロール用アプリを使い、ダブルブラインドで行われました。現在の処方件数や使用状況は明確ではありません。承認されたばかりのサスメド社のアプリが保険適用になった場合も含め、アプリの処方に関する公的なデータは来年初めくらいまでに一部は出てくるはずです。
日本ではスマートフォンに入れるアプリが薬事承認・保険適用されましたが、ヴァーチャルリアリティ(VR)用ゴーグルを使う治療用アプリも開発が進んでいます。対人恐怖症などでは没入感を生かしたトレーニングのような、スマートフォンではできない治療が可能です。
このように治療用アプリは、慢性の病気・症状で行動変容やトレーニングが必要な領域で効果を上げると期待されています。また、体への直接的な副作用がないのもメリットです。
一方で、治療用アプリに懸念がないわけではありません。
1つはアプリにお金を払う価値を患者さんが感じられるかどうかという点です。「CureApp SC ニコチン依存症治療アプリ及びCOチェッカー」は禁煙補助薬を除いて1か月に3割負担で7620円かかります。「CureApp HT」は3割負担で半年で1万5000円ほどです。治療用アプリは医療機器のため患者さんに直接一切の広告ができず、患者さんにどのように価値を伝えていくかは難しいところです。
もう一つは処方した医療機関で患者さんにアプリの使い方を指導する必要があることです。アプリのダウンロード、処方コードの入力などを教える余力が医療機関にあるかどうか。
とはいえ、医薬品と比べると、治療用アプリは研究開発費や臨床試験の費用も安くなるのが特徴です。日本でも保険適用となっている2つの治療用アプリが普及し、効果が示されてくれば、承認へのハードルは以前よりは下がるでしょう。現在、医薬品や医療機器の企業だけでなく、異業種の参入も始まっています。2023年末くらいにはサスメドの新たな治療用アプリも保険で使えるようになると予想されます。
医薬品や医療機器と同様、競合のアプリも出てくるはずです。年齢や性別、好みによって使い分けられる時代が来るかもしれません。また、キャラクターが育つ、使うとポイントがたまるといった医薬品や医療機器には絶対できないエンターテインメント性が付加されるものもありえます。そうなると患者さんが積極的に治療に参加することにつながるのではないでしょうか。
今やデジタル医療の情報は、何を採り入れ、何を入れないのかの経営上あるいは診療上の判断に欠かせないものとなっています。医療者には実際に治療用アプリを処方するか否かに関わらず、このような動きを知っておいていただければと思います。