A PCR test performed to diagnose a new coronavirus infection. People who are suspected of being infected or who are regarded as key workers are required to be actively tested, but there is a limit to the accuracy of PCR tests, and caution is required when making positive and negative decisions. .. We have summarized the issues as a test for new coronavirus infections.
PCR is an abbreviation for polymerase chain reaction, and is a technology that amplifies a specific region (target DNA) of a DNA sequence by millions to billions of times using an enzyme called DNA polymerase. Since a small amount of DNA sequence of only a few molecules can be increased to a few mg, it becomes possible to study the target DNA in detail.
PCR is a technology invented by Kary Mullis of the United States in 1983 and put into practical use in 1986. Maris was awarded the Nobel Prize in Chemistry in 1993 for his contributions to promoting research in a wide range of fields including molecular biology, genetics, medicine, agriculture, and forensics dealing with DNA.
In PCR, first, the target DNA to be amplified is determined in the DNA sequence. Next, we design two synthetic DNAs called "primers" that specifically bind to both ends of the target DNA. This primer serves as a starting point for the synthesis of new target DNA. Then, a DNA polymerase that catalyzes DNA synthesis is prepared, and the sample DNA, a primer, and the DNA polymerase are added to the reaction solution so that the DNA polymerase can work in the optimum environment. Usually, a microtube of a test kit of a test company containing a reaction solution or DNA polymerase is used.
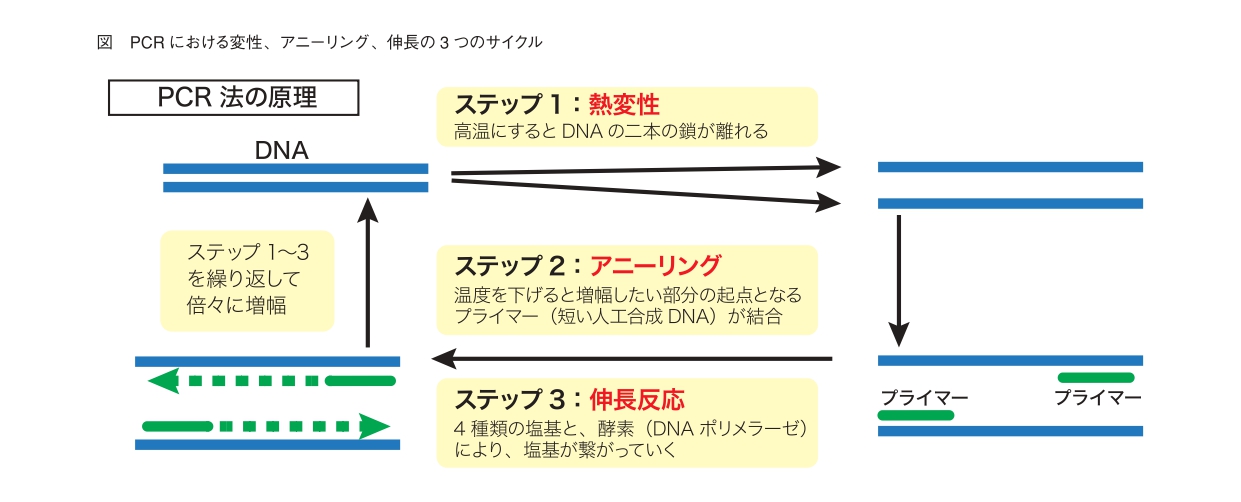
By placing this microtube in a constant temperature bath called a thermal cycler and repeating the three cycles of thermal denaturation (94 to 96 ° C), annealing (55 to 60 ° C), and elongation reaction (70 to 74 ° C), the target DNA is exponential. It will be amplified functionally. At the stage of heat denaturation, the two strands of the heated DNA separate into one strand each. In annealing, the temperature is gradually lowered, and as a result, the primer previously placed in the reaction solution binds to the target DNA of the DNA strand that has become single-stranded by heat denaturation. Then, in the extension reaction, by raising the temperature again, the DNA polymerase acts to synthesize a new DNA molecule. In this way, the DNA strand starting from the primer becomes a "template", and the paired strand is created (Fig.).
PCR is a technique for amplifying target DNA, but it cannot amplify RNA. Therefore, by synthesizing DNA from RNA using reverse transcriptase (RT) and amplifying the DNA by PCR, it has become possible to detect even virus RNA. This is RT-PCR. The test currently performed in Japan for the new coronavirus, which is a type of RNA virus, is a test using this RT-PCR. The three cycles are identical to DNA PCR testing.
There are two methods for RT-PCR testing (hereinafter referred to as PCR testing) for the new coronavirus. One is a method based on the "Pathogen Detection Manual" prepared by the National Institute of Infectious Diseases (Infectious Disease Research Institute), and the other is a sample direct PCR method that simultaneously performs reverse transcription of viral RNA and real-time PCR directly from the sample. be.
The infection laboratory method and the sample direct PCR method have the same purpose but different methods. First, the amplification location of the target RNA is different. In the Infectious Diseases Research Institute, RNA sequences at two locations specific to the new coronavirus called N set and N2 set are amplified and detected. On the other hand, the direct sample PCR method is required to propagate and confirm RNA sequences in two locations specific to the new coronavirus called N1 and N2 defined by the Centers for Disease Control and Prevention (CDC). It is amplified as a thing).
The sample pretreatment process is also different. In the Infection Research Method, it is necessary to extract and purify RNA for about 30 minutes using a special kit after collecting samples (nasopharyngeal swabs and saliva), and it takes more time if the number of samples is large. .. This method has a problem that a certain degree of proficiency is required in the RNA extraction and purification process. In the sample direct PCR method, it is possible to amplify the target sequence by using a primer / probe without performing such a series of RNA extraction / purification operations. Therefore, the new coronavirus can be detected directly from the sample, and the burden on the operator can be greatly reduced. There is.
If RNA specific to the new coronavirus is detected in the sample by the PCR test, the donor of the sample is a person who is positive for the PCR test. Currently, positive people are reported as "infected people", but many researchers question this point.
First of all, there is the problem of the accuracy of PCR tests. In the PCR method, false positives (determined to be positive even though it is not really a new coronavirus infection) and false negatives (determined to be negative even though it is actually a new coronavirus infection) may occur depending on the conditions of sample collection and sample storage. .. False negatives may occur at the stage of the PCR test described above, and may occur even when a worker who is not familiar with the sample collection technique cannot collect a sample well. False positives have various problems, as will be described later. For these reasons, the sensitivity of the PCR test (the rate at which the PCR test is positive due to infection with the new coronavirus) is considered to be about 70%, and it is necessary to carefully judge and respond to the test results. To.
More problems have been pointed out with regard to false positives. In the first place, many infectious disease specialists emphasize that "a person who has a positive PCR test = a person who is not infected with the new coronavirus". First, there are many asymptomatic people among those who test positive, but these are not infected people. "Infection" is established only when the new coronavirus invades the body and proliferates inside the cells, but humans have an immune function that protects themselves from these pathogenic microorganisms, so even if they inhale the virus, they are not always infected. do not have. However, the PCR test used for the new coronavirus may be positive if the inactivated virus floating in the air happens to be inhaled and is mixed with even a few samples of nasal mucosa and saliva. high.
In addition, the RNA sequence that is specific to the new coronavirus and is the target of PCR testing may also be present in other viruses, and is included in the attached document of the PCR testing kit that was used overseas in the past. , There was a description that the conventional coronavirus, rhinovirus, adenovirus, etc. were also positive.
Based on these, the CDC is based on the fact that the PCR test is a test that amplifies a specific region of a certain gene and does not detect the presence of the virus. Therefore, based on the positive result of the PCR kit test, a new type of coronavirus infection. It is said that it should not be used as a basis for treatment.
Performing a PCR test on a person with symptoms such as fever is an important tool for a doctor to make a definitive diagnosis, but careful judgment and response are required, keeping in mind that positive = not necessarily a person infected with the new coronavirus. ing.
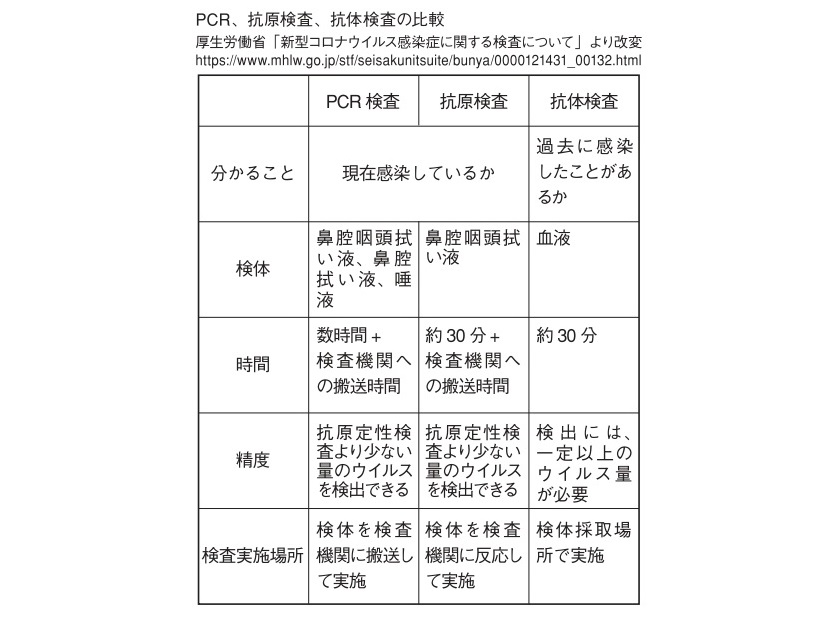
Currently, there are PCR test, antigen test, and antibody test as test methods for new coronavirus.
As explained in the text, the PCR test is a method of reverse transcription of a small amount of viral RNA fragments into DNA and amplification and detection of the DNA in order to determine whether the virus currently exists in the body. The specimen is mucus or saliva wiped from the back of the nose.
抗原検査は、新型コロナウイルスに対する抗体を用いて抗原(ウイルス表面のタンパク質の断片)を検出する検査。検体はPCR検査と同じく鼻粘膜や唾液。高価な機械や訓練、労力がなくても抗原の検出が可能で、短時間で新型コロナウイルスの新規感染を診断できるとされる。ただし、PCRに比べ、検出にはより多くのウイルス量が必要で、精度は劣る。
The antibody test is a test to check if you have been infected in the past or if you have already acquired an antibody that resists the virus, and the sample is blood. The major difference is that the PCR test and the antigen test detect the virus and its fragments, whereas the antibody test detects the antibody produced in the body of a person infected with the virus.
For the convergence of new coronavirus infections, it is important to properly use these test methods for diagnosis and treatment.