ASOURCE®NAVI

公開日:2022.12.28
コロナ禍で新型コロナウイルス感染症のワクチンや治療薬が従来より数年早い発売が可能となった特例的な承認制度が注目されました。これまで日本は医薬品の審査に時間がかかり、新薬の登場が欧米より数年遅れるといわれていましたが、最近では新しい制度の導入などでタイムラグが大幅に解消されています。そこで、医薬品の薬事承認の仕組みについて、最近の動向も踏まえご紹介します。
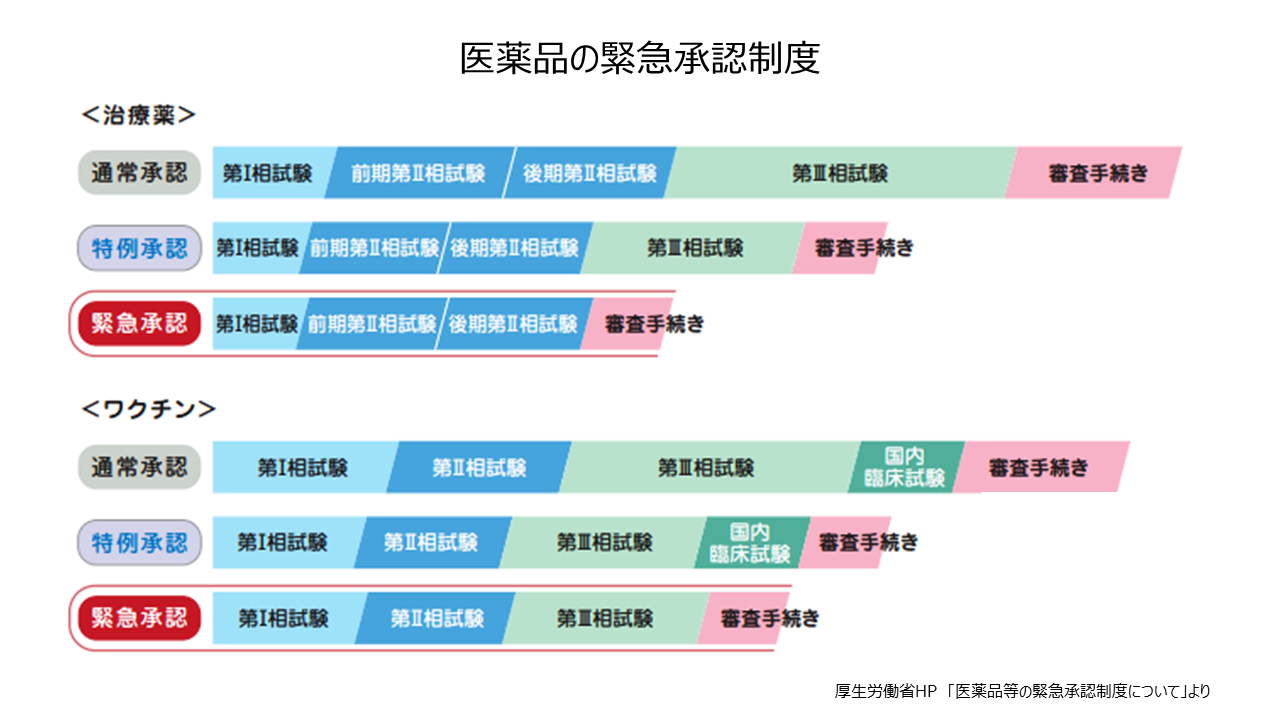
医療用医薬品は薬機法(医薬品、医療機器等の品質、有効性及び安全性の確保等に関する法律)などに基づいて審査・承認されますが、承認されるまでにいくつかの過程があります。まず、医薬品の候補となる物質について、動物や細胞を使って薬効や毒性などを検討します。次に少数の健康成人に投与して、吸収や代謝、排泄などを検討します(第I相試験)。さらに少数の対象疾患患者に投与して、有効性や安全性を調べ(第II相試験)、のちに多数の患者に投与して、有効性や安全性を検討します(第III相試験)。第II相、第III相試験は、二重盲検試験とランダム化比較試験を実施します。第III相試験は約200人以上が対象となり、数年程度の期間がかかります。
臨床試験結果などをまとめた製薬会社は、新薬の販売承認を取得するために、厚生労働省に対して承認申請を行います。承認許可を出す機関は厚生労働省ですが、承認するための審査を行う機関は医薬品医療機器総合機構(PMDA)です。PMDAで、新薬の資料・臨床試験結果をもとに審査が行われ、その後、厚生労働大臣の諮問機関の薬事・食品衛生審議会で、PMDAが作成した審査報告書をもとに審議されます。そして、医薬品としてふさわしいという結論が出た場合、厚生労働大臣による販売許可が下ります。承認申請から承認取得に至る期間は1~2年ほどとなっています。

日本で2021年に承認された新医薬品の品目数は135品目で、2020年より10品目増加し、2010~2020年の平均承認品目数の118品目より多い結果でした。2021年に承認された全品目の審査期間の中央値は9.9か月と、2020年より0.8か月短く、審査期間が大幅に短縮した2011年以降は10か月前後で推移しています。
医薬品の審査には、新薬発売までのスピードを早めるために条件に基づいたうえでの例外的措置が適用されることがあります。今年5月に薬機法が改正され、「緊急承認制度」が創設されました。第II相試験相当の臨床試験で安全性が確保され、有効性が推定されれば、緊急に承認できるというものです。ただし、承認後2年間のうちにその有効性を証明する臨床試験データを示す必要があります。緊急承認制度による第1号は国産初のコロナ治療薬ゾコーバです。また、「特例承認制度」は2010年の新型インフルエンザ感染拡大時、欧州からワクチンを緊急輸入する際に創設されました。感染症などの健康被害の拡大を防ぐため、医療技術が日本と同程度の欧州や米国で使用されている医薬品について、有効性と安全性の両方を早急に確認し、迅速な承認を行うものです。このほか、優先審査の制度として以下のものがあります。①世界に先駆けて革新的な医薬品・医療機器などを日本で初めて実用化するために創設された「先駆的医薬品等指定制度(先駆け審査指定制度)」②患者数が5万人未満で医療上特にその必要性が高い疾患が対象となる「希少疾病用医薬品指定制度」③患者数が少ないなどの理由で臨床第Ⅲ相試験を実施することが難しい医薬品・医療機器などについて、発売後に有効性・安全性を評価することを条件に承認する「条件付き早期承認制度」④培養細胞などで体の構造や機能を再生するものや、遺伝子治療に用いる製品を対象とした「再生医療等製品の条件・期限付き承認制度」。こうした制度は日本国内での新薬開発を企業が優先しやすくなり、恩恵を受ける患者の増加につながるものと思われます。
メディアスグループは、医療機器の販売を中心とした事業を展開しています。医療に携わる私たち(Medical+us)は、医療現場や人々の健康的な明日へ役立つ情報をお届けする情報発信源(Media)の役割も果たしていきたいと考えています。