ASOURCE®NAVI

公開日:2022.02.01
5歳から11歳までの子どもへの新型コロナウイルスのワクチン接種が今年3月から始まる見通しとなりました。オミクロン株が驚異的な速さで感染拡大するなか、自分の子どもにワクチンを接種させるべきかどうか悩む保護者は少なくないと思われます。ワクチン接種のメリットとデメリットを正しく理解して判断することが重要といえます。
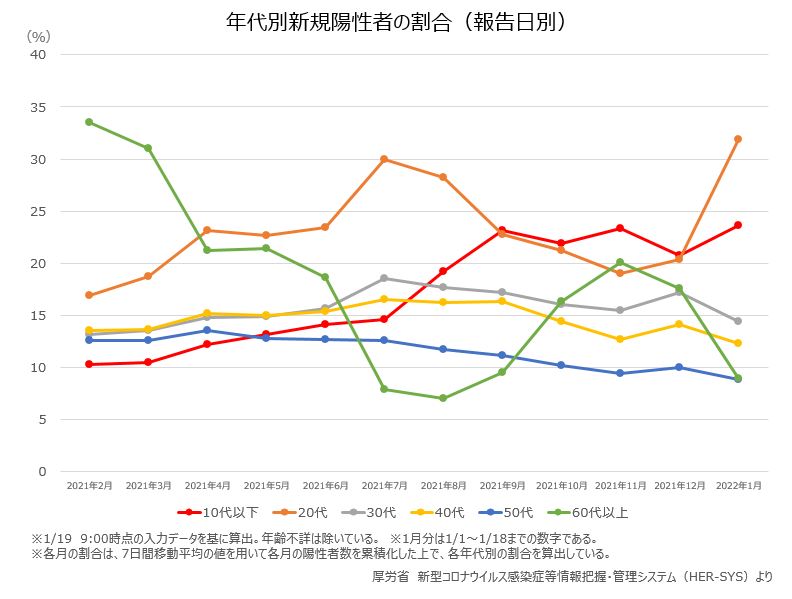
厚労省によると、10歳代以下の新型コロナの新規感染者数は昨年12月から増加に転じ、1月12〜18日で10歳代の新規感染者は2万6,560人、10歳未満は1万3,050人で、全体の24%を占めるまでになっています。オミクロン株の流行が先行する沖縄県では、1月上旬までの20代を中心とした爆発的な感染拡大は収まりつつありますが、小児や高齢者へ感染拡大し始めています。1月17〜23日には全年齢層のなかで10歳代の感染、10歳未満の感染がそれぞれ約15%を占めています。
5〜11歳の新型コロナウイルス感染症患者の大多数は軽症と考えられています。国立成育医療研究センターと国立国際医療研究センターが合同で行った小児コロナ患者の研究では、2020年1月〜2021年2月に全国約570施設で報告されたコロナ感染により入院した18歳未満の小児患者1,038人を対象に検証しました。その結果、無症状の患者は308人(30%)、何らかの症状があった患者は730人(70%)で、症状のあった患者のうち酸素投与を必要としたのは15人(2.1%)、死亡例は0人で、多くは軽症であることがわかりました。38℃以上の熱が出た患者は、症状のあった患者のうち約10%でした。ただし、入院期間は8日と比較的長期に及んでいました。また、厚労省の新型コロナウイルス感染症等情報把握・管理システム(HER-SYS)によると、2021年4月〜12月の5〜11歳の新型コロナ感染者は6万1967人で、そのうち中等度以上は171人(約0.3%)、重症25人(0.04%)、死亡0人でした。
子どもへのワクチン接種については、昨年5月に接種対象が12歳以上となり、今年1月21日に5歳から11歳までも対象に加わることが正式に承認されました。5〜11歳用のワクチンはファイザー製で、12歳以上のものとは異なり有効成分量は3分の1で、3週間の間隔を空けて2回接種します。米国の5〜11歳の子どもを対象とした臨床試験では、偽薬を投与した750人のうち16人が発症しましたが、ファイザー製の通常の3分の1量を2回投与された約1,500人のうち発症したのは3人で、発症予防効果は90.7%でした。ただし、臨床試験が実施されたのは主にデルタ株が流行している昨年で、オミクロン株への効果について反映されていないとされます。
イスラエルの最新の研究では、ワクチンを2回接種した5歳から11歳の子どもたちは、未接種の子どもたちと比べてオミクロン株に感染するリスクが約2倍低いことが明らかになっています。イスラエル政府がワイツマン研究所、ヘブライ大学、シェバ医療センター・ゲルトナー研究所と共同で実施した調査によると、昨年12月25日から今年1月16日の間に5〜11歳の未接種の子どもが1日平均で10万人あたり約260人が感染していたのに対して、ファイザー製ワクチンを2回接種した子どもでは約120人の感染にとどまり、ワクチン接種がオミクロン株に対して発症予防効果を発揮することが判明しました。
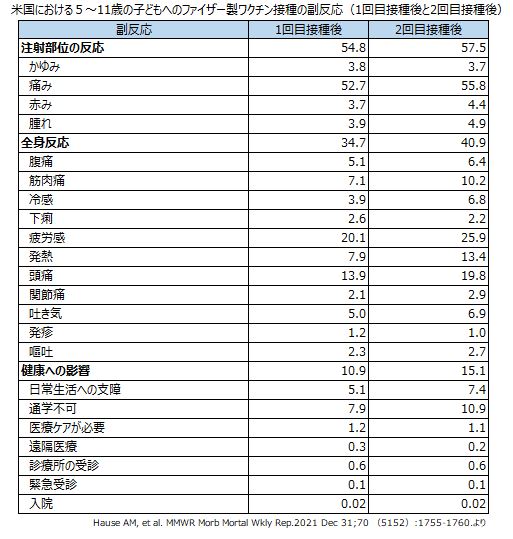
ワクチン接種後の副反応など安全性に関するデータも集積されつつあります。米国では、2021年11月3日から12月19日までに5〜11歳の子どもに約870万回のファイザー製ワクチンが接種され、登録された約4万2,500人の副反応について検討されました。それによると、2回接種後1週間以内の副反応としては、接種部位の痛み55.8%(1回接種後52.7%)、疲労感25.9%(同20.1%)、頭痛19.8%(同13.9%)、発熱13.4%(同7.9%)筋肉痛10.2%(同7.2%)などでした。接種後に学校への出席が困難となる頻度は高くなく、医療ケアが必要となるケースは稀でした。また、同時期に、米国の予防接種安全監視システム(VAERS)には、4,249件の副反応の疑い症例が報告され、このうち97.6%が軽症でした。重症として報告された100件中多かったのは発熱29件、嘔吐21件などでした。11件が心筋炎と判断されましたが、全員回復しました。5〜11歳の小児では16〜25歳の人と比べて一般的に副反応症状の出現頻度は低いとみられています。最も副反応としての接種部位の痛みや発熱、頭痛、倦怠感などは、この年齢に接種される他のワクチンと比べてその発現率は高いと想定されています。
我が子にワクチンを接種させるべきか否か、思い悩む保護者は多いと思われます。日本小児科学会は「今後、全年齢において感染者が増加した場合には、ワクチン未接種の小児が占める割合が増加し、小児の中等症や重症例が増えることが予測され、基礎疾患のある小児へのワクチン接種でコロナの重症化を防ぐことが期待される。また、健康な子どもでも接種の意義がある」とし、きめ細かな対応が必要だとしています。また、日本小児科医会は「年齢が低い小児であっても感染してしまった場合の他者への感染リスクの増大、10日以上にもわたる行動制限の必要性と困難性などを考慮すると、子どもたちの心身への影響は計り知れない」とし、十分な議論と準備の上での接種を求めています。ワクチンのメリットとリスクを正しく理解して判断することが必要とされます。
メディアスグループは、医療機器の販売を中心とした事業を展開しています。医療に携わる私たち(Medical+us)は、医療現場や人々の健康的な明日へ役立つ情報をお届けする情報発信源(Media)の役割も果たしていきたいと考えています。