ASOURCE®NAVI

公開日:2021.02.02
追記(2021.3.8): 記事は2021年2月2日に投稿時の情報です。その後2月下旬にファイザー社は、−75℃の超低温冷凍から-15~-25℃の温度帯での冷凍に移した後、14日間の保存ができるとする新たなデータをアメリカ食品医薬品局(FDA)に提出し、認められました。日本国内でもこのほど、同様な保管が可能となり、添付文書が改訂されました。これにより、医療施設にある標準の医療用冷凍庫で対応が可能となります。また、保管のための温度要件を緩和する取り組みは引き続き行われているため、今後更に輸送管理がしやすくなる見通しです。
新型コロナウイルスワクチンは数種類が開発の最終段階を迎え、その一部は承認待ちとなっていますが、今課題となっているのが、ワクチンを入れた小さなガラス容器をどうやって低温を保ったまま接種が実施される医療機関や施設まで運び届けるかです。
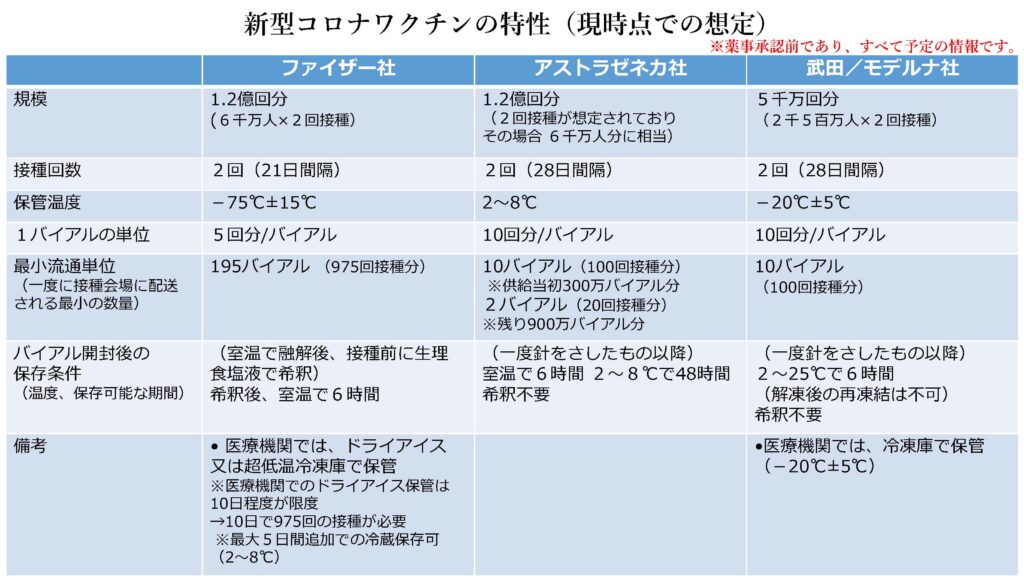
正式契約を締結し、ワクチン供給を受けることが決まっているのは、米ファイザー、米モデルナ、英アストラゼネカの3種類です。もっとも早く接種が可能とみられるファイザー製のワクチンの保管温度は、−75℃(±15℃)。モデルナ製の保管温度は−20℃(±5℃)で、それぞれ6ヶ月間の保管が可能とされます。
いずれもmRNAと呼ばれるタイプのワクチンで、構造が壊れやすい遺伝子で作られているため、常に超低温に保たれなければ接種しても効果が失われる恐れがあるといわれています。アストラゼネカ製の保管温度は2〜8℃となっており、冷蔵は必須であるものの、冷凍の必要はありません。
厚労省は、ワクチン接種に必要な物資と物流を整備するため、−75℃のディープフリーザー(超低温冷凍庫)、−20℃のディープフリーザー各10,000台を確保したとされます。ディープフリーザーは国内メーカー4社が供給。設置が容易に可能な70〜90 ℓの小型タイプで、電源は100V。性能は各社同様のものとされます。ディープフリーザーは人口を基に各自治体に割り当てる方針といわれています。
ファイザー製のワクチンは海外工場から空輸された後、国内倉庫のディープフリーザーで保管され、医療機関や接種会場には約1,000回接種分を1単位として、トラックなどで保冷ボックスとドライアイスを利用して輸送します。保冷ボックスは、ドライアイスの詰め替えにより配送から約10日程度保管可能で、冷蔵保管の場は、約5日間保管できます。
モデルナ製ワクチンは、武田薬品工業が国内受け入れ拠点・全国配送用分置倉庫においてリファーコンテナ(冷凍・冷蔵貨物の輸送に使用される特殊コンテナ)で保管し、全国の卸倉庫・支店までトラック輸送し、ディープフリーザーで保管。医療機関・接種会場には車載可能なディープフリーザーを使用、100回接種分を1単位として届けます。モデルナ製は通常の冷凍庫の温度で10日ほど保管できます。
アストラゼネカ製ワクチンは、インフルエンザワクチン並みに冷蔵庫保管し、国内倉庫から卸倉庫・支店には保冷トラックで輸送。医療機関・接種会場には保冷ボックスで届ける予定です。
最終配送体制としては、厚生労働省が指定した医薬品卸40社が受け持ち、新型コロナウイルスのワクチンを接種施設まで届けることになっています。東京都では、メディセオ、アルフレッサ、スズケン、東方薬品の大手4社、大阪府ではこの大手4社にケーエスケーを加えた5社、愛知県では、大手4社に中北薬品を加えた5社が担います。なお、ファイザー製ワクチンについては、-70℃での管理が必要なため、製薬企業から医療機関に直接配送されます。
ワクチンによって保管温度が異なることから、医療機関では、ワクチンの保管作業が煩雑になることが懸念されています。
新型コロナウイルスワクチンの接種体制確保について 自治体説明会➀より
厚生労働省健康局健康課予防接種室(令和2年12月18日)
メディアスグループは、医療機器の販売を中心とした事業を展開しています。医療に携わる私たち(Medical+us)は、医療現場や人々の健康的な明日へ役立つ情報をお届けする情報発信源(Media)の役割も果たしていきたいと考えています。