ASOURCE®NAVI
公開日:2022.06.07
ノババックス社が開発した新型コロナウイルスワクチンの接種が始まっています。「組換えたんぱくワクチン」というもので、国内で4番目のワクチンです。他のコロナワクチンよりも副反応が少ないとみられ、新たな選択肢となりそうです。
ノババックス製ワクチンは、18歳以上を対象に使用されるもので、接種期間は、1回目から3週間空けて2回目を接種、さらに6カ月経過すれば3回目の接種ができるというものです。これまでのファイザーやモデルナのワクチンは、「mRNAワクチン」といい、遺伝子を入れることで、抗体を作るために必要な物質を体内で作る仕組みです。これに対して、ノババックス製ワクチンは、「組換えたんぱくワクチン」といい、抗体を作るために必要な物質そのものを体内に入れる仕組みです。
ノババックスワクチンの効果については、米国とメキシコで2020年12月から2021年2月までに約3万人を対象に実施された臨床試験では、2回接種後の発症予防効果は90.4%、中等度や重症を防ぐ効果は100%でした。他のコロナワクチンの治験の発症予防効果は、ファイザー製95%、モデルナ製94.1%、アストラゼネカ製62.1〜70.4%です。いずれも治験時点での流行株はアルファ株やベータ株でしたので、オミクロン株に対しては効果が下がると考えられています。
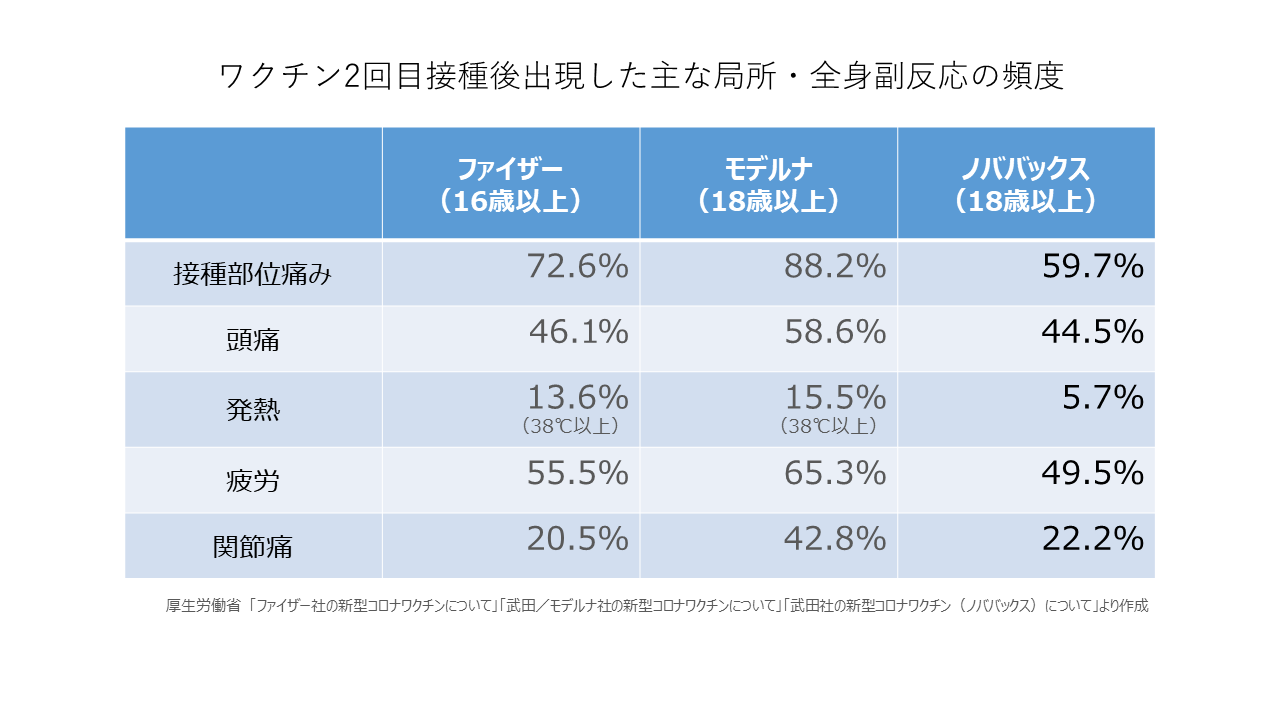
副反応については、米国とメキシコで行われた治験では2回目接種後に発熱した人はノババックス製で5.7%、頭痛は44.5%、疲労は49.5%、関節痛は22.2%、接種部位の痛みは59.7%などでした。ファイザー製の治験では、発熱13.6%、頭痛46.1%、疲労55.5%、関節痛20.5%、接種部位の痛み72.6%など、モデルナ製の治験では発熱15.5%、頭痛58.6%、疲労65.3%、関節痛42.8%、接種部位の痛み88.2%などで、ノババックス製の方がファイザー製やモデルナ製よりも発生頻度が低い傾向にありました。
英国で行われた交互接種試験によれば、ファイザー製ワクチン2回目を接種した人に対し、ノババックス製ワクチンの追加接種(全量)を行ったところ、髄膜炎菌ワクチンを接種した人と比較して、追加接種28日目の抗体が4.78倍上昇しました。デルタ株に対する中和抗体も4.94倍上昇しました。交互接種の副反応としては、114人中44人に副反応がみられましたが、重篤なものはありませんでした。
副反応などを懸念し、これまでのワクチン接種を見送ってきた人たちにとってノババックス製は新たな接種の動機となる可能性があります。
メディアスグループは、医療機器の販売を中心とした事業を展開しています。医療に携わる私たち(Medical+us)は、医療現場や人々の健康的な明日へ役立つ情報をお届けする情報発信源(Media)の役割も果たしていきたいと考えています。