ASOURCE®NAVI
公開日:2021.06.11
実用化が待たれる新型コロナウイルスワクチンの国内開発が新たな問題に直面しています。海外産のワクチン接種が広がり臨床試験の実施が難しくなっていることや緊急時の医薬品の承認制度の問題が障壁となっているからです。治験の方法や承認プロセスの見直しが迫られています。
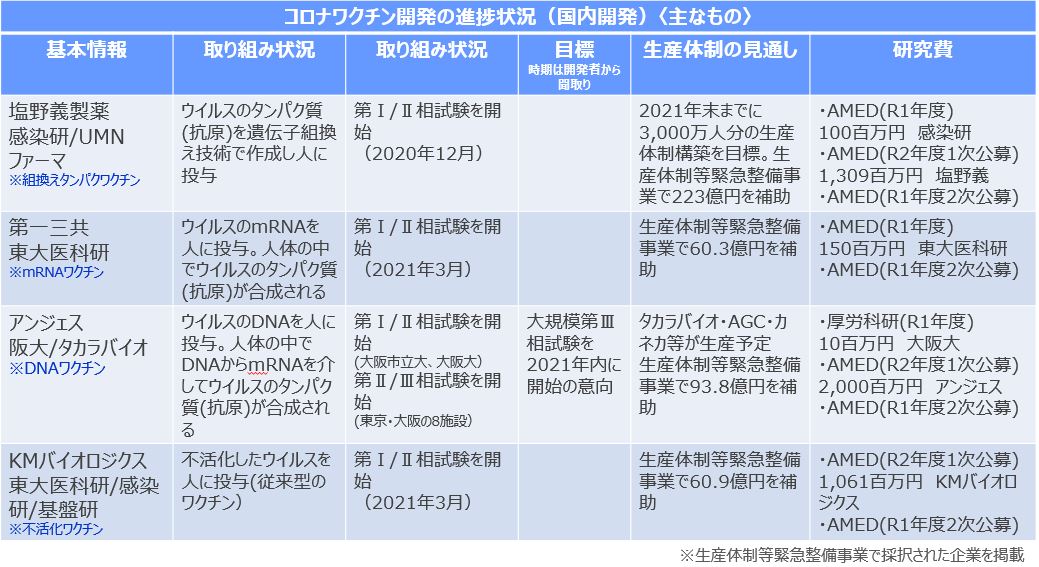
新型コロナウイルス感染症の流行の収束の切り札とされるワクチンの国内開発・生産が海外に比べて大幅に遅れています。現在、国内で新型コロナウイルスワクチンの治験が行われている製薬企業は4社です。アンジェスはDNAワクチンの開発で第II/III相試験。塩野義製薬は組換えタンパクワクチン、第一三共はmRNAワクチン、KMバイオロジクスは不活化ワクチンーの開発で、それぞれ第I/II相試験を実施しています。
厚生労働省 第5回医薬品開発協議会(2021年5月25日)資料より
ワクチンは、治療薬と異なり、健康人を対象としているので、後発のワクチン開発企業にとっては、既に先発のワクチンが使用されているなかで薬事承認に向けた最終段階の大規模第III相試験において二重盲検試験のために数万人の被験者を確保することは難しく、多額の治験費用がかかることも開発を妨げているとの指摘があります。また、新型コロナウイルス感染症は死亡リスクもあり、すでに有効性の高いワクチンが存在するため、偽薬の投与に倫理的な問題がつきまといます。こうしたことから、すでに効果が確認されているワクチンの中和活性データと比較するなど発症予防試験以外の方法でワクチンの効果を確認することも検討されています。
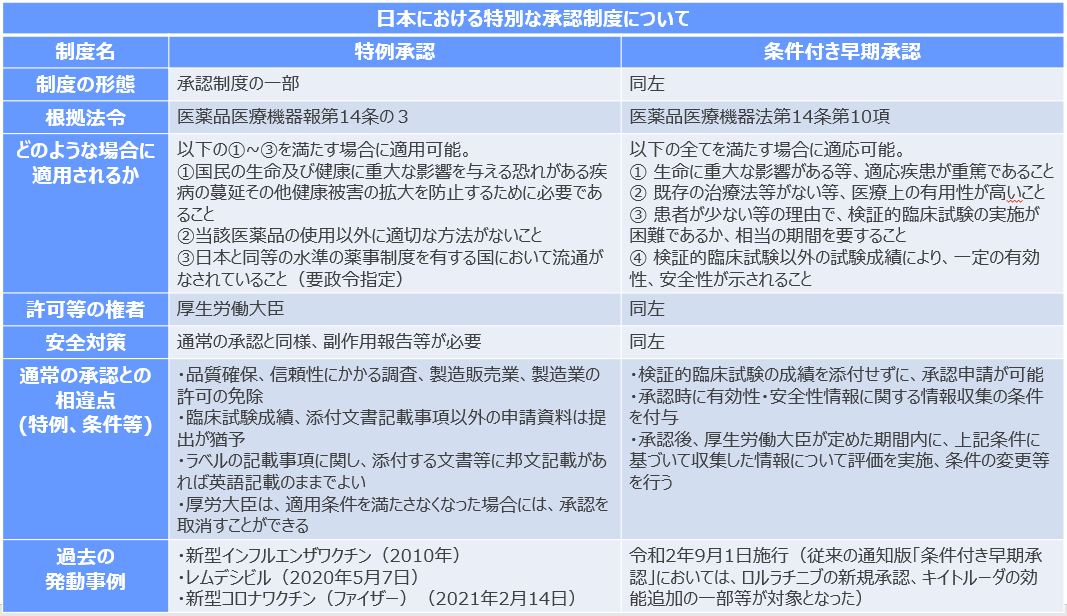
新型コロナウイルスワクチンについては、治験の方法だけでなく、承認プロセスの見直しの必要性も指摘されています。海外の国では、今回のような新型コロナウイルスの流行という緊急時には通常時とは違って、新しい薬剤の承認を迅速に勧められる仕組みを整えています。米国では緊急使用許可(EUA)という制度により米食品医薬品局(FDA)が緊急時にワクチンの生産や承認を早めることが可能となっています。すなわち、緊急時に限り、未承認でも新型コロナウイルス感染症の流行が収束するまでとの条件付きで一時的に認めるというものです。第III相試験は必要ですが、通常よりも短い経過観察期間で調べた中間データでも申請できます。FDAは新型コロナウイルスワクチンの申請から1ヶ月未満でEUAを出しています。
厚生労働省 第4回医薬品開発協議会(2021年4月16日)資料より
日本の場合、現在のところ緊急時であっても承認していないワクチンや治療薬の一時的な使用を認める規定はありません。通常、承認申請から承認されるまで約1年かかりますが、ファイザー製、モデルナ製、アストラゼネカ製などのワクチンは薬機法14条に定める「特例承認」を適用しました。緊急時にそのワクチンを使用するほかに適切な方法がなく、海外で実績があるなどの条件を満たせば手続きを簡略する規定です。これらのワクチンの場合、海外で使用が始まっていたため、優先的に審査して使用が認められました。しかし、この特例承認でも国内の治験手続きは省けません。
こうしたことから、緊急対応策として、患者数が少なく治験が難しい希少疾患の医薬品などを念頭に制度化されている「条件付き早期承認」をワクチンに適用拡大する提案も出されています。すなわち、一定の安全性と有効性を確認した上でフェーズIII前に承認し、市販後の追跡調査で確認するというものです。
国は新型コロナウイルスワクチンを「国民の命を守る安全保障の要」と位置付け、国家戦略として国産ワクチンの開発・生産の支援を行っていく構えです。そのために現在、大規模治験のあり方や薬事承認手続きの簡略化の検討を進めています。
メディアスグループは、医療機器の販売を中心とした事業を展開しています。医療に携わる私たち(Medical+us)は、医療現場や人々の健康的な明日へ役立つ情報をお届けする情報発信源(Media)の役割も果たしていきたいと考えています。