ASOURCE®NAVI

公開日:2021.10.27
世界的にみて10代前半の子どもに対するコロナワクチン接種には明確なコンセンサスはないとされ、子どもを対象にしたコロナワクチン接種への対応については国によって対応が異なっています。
日本では12〜15歳の子どもを対象にコロナワクチンの2回接種が実施されていますが、12〜19歳の接種率は1回目約67%、2回目約48%となっています(10月25日時点)。海外では、フランス(12〜17歳)では、1回目66%、2回目52%。デンマーク(12〜15歳)とスペイン(12〜19歳)ではほとんどの子どもたちに1回目の接種が実施されています(9月時点)。米国(12〜17歳)では1回目42%、2回目32%(7月末時点)となっています。
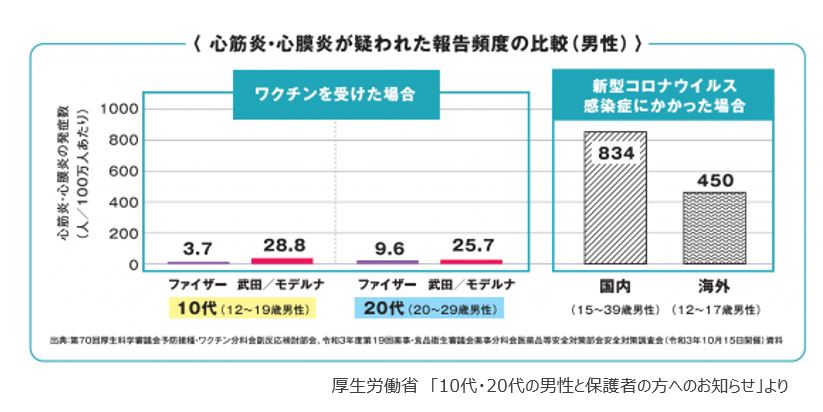
日本国内で使われている新型コロナワクチンは3種類ありますが、12歳以上の子どもが打てるのはファイザー製かモデルナ製のmRNAワクチンです。厚生労働省は10月15日、10~20代の男性は、モデルナ製のワクチン接種後に心筋炎、心膜炎の症状が出る割合が比較的高いとし注意喚起し、心配な場合は、1回目にモデルナ製を接種した人が、2回目にファイザー製を選ぶことも認めることにしました。心臓の筋肉の炎症を心筋炎、心臓を包む膜の炎症を心膜炎といい、症状としては、胸の痛み、息切れ、動悸などが現れます。12〜19歳男性に限れば、100万人接種あたりの心筋炎・心膜症の発症数は、モデルナ製が28.8人でファイザー製の3.7人より7.8倍高いという報告が示されています。このため、海外では、10月6日にスウェーデンでは30歳以下、デンマークは18歳以下へのモデルナ社ワクチン接種の一時停止を発表しています。
一方、英国は、今年9月に12〜15歳へのワクチン接種は1回だけとし、2回目は詳細なデータが集まるまで早くても来年の春までワクチン接種を見合わせるよう勧告しています。その理由としては、子供たちのウイルス感染のリスクは非常に低く、ワクチンを接種してもわずかなメリットしか得られないからだとしています。今回の決定は、ファイザー製とモデルナ製のワクチンには、心筋炎や心膜炎という極めて稀な副作用があることを懸念しての措置とされます。
ただし、ワクチン接種後に心筋炎・心膜炎を起こした例のほとんどは軽症例で自然に軽快するとされます。また、ワクチン後の心筋炎・心膜炎は新型コロナに感染した場合の心筋炎・心膜炎の発生率よりも頻度が低いことも報告されています。
12〜15歳のコロナワクチンの副反応ついては、アジア人を含む12歳から15歳までの1,131人を対象にファイザー製ワクチンを投与された臨床試験の結果が米国の臨床試験グループより報告されています(The New England Journal of Medicine: 2021年7月15日号)。それによると、38℃以上の発熱は、2回目投与後に20%(1回目投与後10%)で認められました。投与翌日に40℃以上の発熱が1人にみられました。解熱剤の使用頻度は51%(同37%)で、ほとんどの人は解熱剤投与で回復し、数日以内で治りました。また、注射部位の痛みは79%(同86%)にみられましたが、激しい痛みを訴えた人は1.5%にとどまりました。その他の投与後の副反応の内訳としては、2回目後は倦怠感66%(1回目後60%)と頭痛65%(同55%)が多く、続いて悪寒42%(同28%)、筋肉痛32%(24%)、関節痛16%(同10%)、下痢6%(同8%)、嘔吐3%(同3%)の順となっています。
このほかの年代の子どもへの接種については、米食品医薬品局(FDA)の諮問委員会は10月26日、ファイザー製ワクチンの緊急使用許可を5~11歳に拡大する勧告を決めました。大人の3分の1の用量で約91%の有効性が確認され、接種による副反応は深刻なものはないことから、接種のベネフィットがリスクを上回ると判断したものです。FDAが許可すれば早ければ11月にも接種が始まる見込みです。一方、モデルナは、同社製ワクチンの6〜11歳への治験で、大人の半分の用量で抗体が大人の1.5倍に増え、副反応は大半が軽度または中程度であることが確認されたため、近くFDAや各国の規制当局にデータを提出するとしています。
日本小児科学会は、健康な子どもへのワクチン接種について、「接種にあたってはメリットとデメリットを本人と養育者が十分に理解していること、接種前・中・後におけるきめ細かな対応を行うことが前提である」としています。
メディアスグループは、医療機器の販売を中心とした事業を展開しています。医療に携わる私たち(Medical+us)は、医療現場や人々の健康的な明日へ役立つ情報をお届けする情報発信源(Media)の役割も果たしていきたいと考えています。