ASOURCE®NAVI

公開日:2021.07.27
新型コロナウイルス対策の柱とされるワクチン接種が進み、終息に向けては特効薬が望まれますが、これまで思うように進展してこなかったのが治療薬の開発です。ここにきて、新型コロナウイルス感染症の治療薬の開発が進み、軽症から重症まで対応できる薬剤が徐々にそろいつつあります。国内では、新型コロナの治療薬として、今年7月中旬までに4つの薬が承認されました。また、いくつかの開発中の治療薬は年内の承認申請を目指しています。
新型コロナ感染の約80%を占める軽症の段階から使用できる薬が中外製薬の「ロナプリーブ点滴静注セット300」「同セット1332」(一般名:カシリビマブ及びイムデビマブ)です。今年7月19日に厚生労働省より特例承認されました。最初から新型コロナの治療を目的として開発された薬剤としては国内初となります。同薬はカシリビマブとイムデビマブの2種類のウイルス中和抗体を同時に点滴投与します。新型コロナウイルスは人の細胞と結合し侵入して増殖しますが、「抗体カクテル療法」と呼ばれるこの治療法では、点滴で投与された2種類の抗体がウイルスと結合することで、細胞への侵入を阻止します。その結果、ウイルスの増殖を防ぎ、重症化するリスクを軽減する仕組みです。対象は年齢や肥満、基礎疾患といった重症化リスク因子を有し、酸素投与が不要な軽症から中等症の入院患者。海外の臨床試験では非投与群と比べて入院または死亡リスクを約70%減少させる結果が得られています。また、デルタ株をはじめとする複数の変異株に効果があることが非臨床試験で確認されています。この薬は昨年トランプ前米国大統領がコロナに感染した際に投与され、話題となりました。
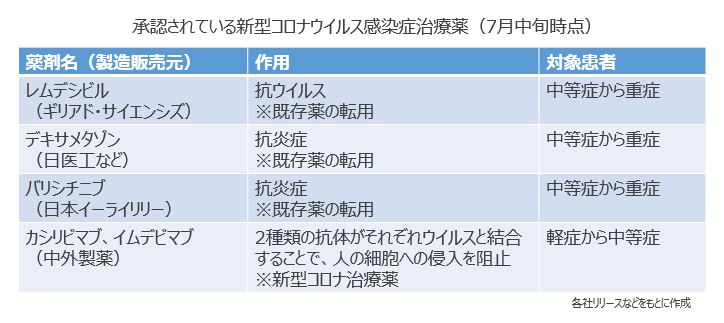
新型コロナの中等症から重症を対象とした薬剤としては、3つが承認されていますが、その多くは他の疾患向けに開発された既存薬が転用されています。ギリアド・サイエンシズのレムデシビルはエボラ出血熱の治療薬として開発されたもので、2020年5月に重症患者を対象に特例承認され、今年1月中等症患者にも投与可能となりました。治験では投与開始から14日目までに回復がみられた患者の割合はレムデシビル群が74.4%、標準治療群では59.0%。また、14日時点の死亡率は、レムデシビル群が7.6%、標準治療群では12.5%でした。日医工などが後発品として製造しているデキサメタゾンは重症感染症や間質性肺炎などの治療薬として承認されているステロイド薬で、昨年7月に中等症から重症の患者を対象に審査・承認なしで認定されました。英国の臨床研究では、人工呼吸器が必要な患者の死亡率は標準治療群が約40%だったのに対しデキサメタゾン群は約29%でした。日本イーライリリーのバリシチニブは関節リウマチの薬で、炎症反応に関わるJAK(ヤヌスキナーゼ)酵素を阻害することで症状を抑えるものです。今年4月に中等症から重症の患者を対象に特例承認されました。新型コロナは重症化すると過剰な免疫反応が起こり臓器障害を引き起こす場合がありますが、同薬は免疫異常による炎症を抑制する作用があります。
このほか、複数の国内企業が治療薬候補の年内の承認申請を目指しています。中外製薬が開発した関節リウマチ薬であるアクテムラは、今年6月に米食品医薬品局(FDA)より人工呼吸器やECMO(体外式膜型人工肺)などを使う重症患者向けのコロナ治療薬として緊急使用許可を取得。日本国内ではすでに治験を完了しており、年内の承認申請を行う運びとなっています。同薬は、過剰な免疫反応を抑制する効果があり、英国の治験では死亡リスクの軽減が認められています。富士フイルム富山化学は開発した抗インフルエンザ薬であるアビガンについて昨年秋に承認申請しましたが、治験の手法の問題などから「有効性の判断は困難」などとして継続審議となっていました。このため、改めて重症化リスクの高い50歳以上の患者を対象に治験を実施し、今秋までに完了する予定です。興和は新型コロナウイルスの増殖を抑える効果や抗炎症作用が確認されている抗寄生虫薬イベルメクチンについて、国内で軽症患者を対象とした治験を行い、年内の承認申請を目指すとしています。更に、7月26日には塩野義製薬が飲み薬タイプの新型コロナウイルス治療薬の臨床試験(治験)を開始したと発表しました。この薬は新型コロナウイルスの増殖に必須の酵素である3CLプロテアーゼを選択的に阻害することでコロナウイルスの増殖を抑制します。治験では健康人を対象に投与し安全性などを検証する予定です。第二段階以降の治験や承認申請の時期は未定としています。
メディアスグループは、医療機器の販売を中心とした事業を展開しています。医療に携わる私たち(Medical+us)は、医療現場や人々の健康的な明日へ役立つ情報をお届けする情報発信源(Media)の役割も果たしていきたいと考えています。